GammaCore is a pain treatment technology that was just approved by the FDA. Find out how the technology works to relieve cluster headache pain today in our review.
What Is GammaCore?
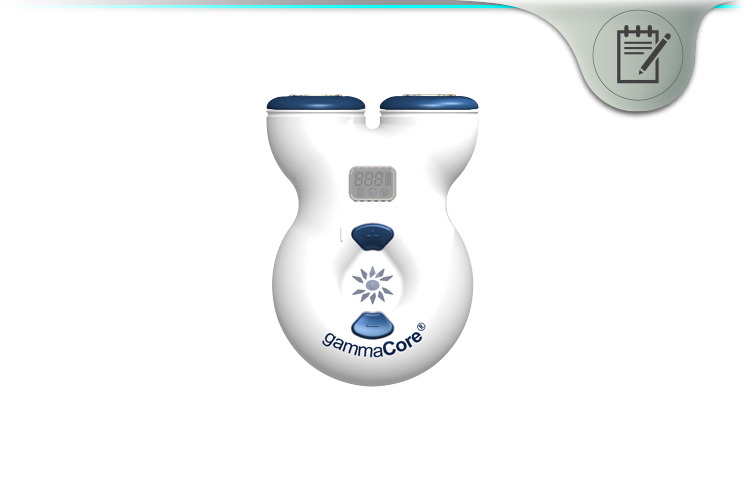
GammaCore is a pain relief technology created by ElectroCore, a neuroscience and technology company. On April 21, 2017, the FDA approved the use of GammaCore for the acute treatment of pain associated with “episodic cluster headache in adult patients”.
GammaCore is the first ElectroCore technology approved for use in the United States. Today, the technology is available and in-use across the European Union and countries around the world – including Canada, Australia, New Zealand, South Africa, and others.
The handheld device can be used at home to relieve cluster headache symptoms. In clinical trials, it was able to relieve pain in as little as 15 minutes in the majority of patients.

How Does GammaCore Work?
GammaCore is a non-invasive vagus nerve stimulator. In clinical trials, it showed an ability to treat pain associated with episodic cluster headaches in adult patients.
The technology works by transmitting a mild electrical stimulation to the vagus nerve through the skin. This leads to reduced pain from cluster headaches.
The device is designed to be portable, handheld, and easy-to-use. You can administer treatment at home. You don’t need to visit a doctor’s office to relieve cluster headaches.
What Are Cluster Headaches?
Cluster headaches are a rare type of headache that causes debilitating pain. Unfortunately for cluster headache sufferers, there are few effective acute therapies.
By releasing GammaCore, the FDA has made “an important advance in the treatment of the pain associated with cluster headache”, explains Stephen Silberstein, MD, Director, Headache Center, Jefferson University in Philadelphia.
“It is a way for patients to treat their symptoms as often as they need to use the device. It does not have the side effects or dose limitations of commonly prescribed treatments or the need for invasive implantation procedures, which can be inconvenient, costly and high-risk.”
The FDA approved GammaCore after it went through two clinical trials to demonstrate its effectiveness.
The GammaCore Clinical Trials
The FDA released GammaCore due to promising results from two trials. Those trials were part of the ACT, or Non-Invasive Vagus Nerve Stimulation for the Acute Treatment of Cluster Headache clinical trial program (the ACT comes from “ACute Treatment” in the name).
Both trials (ACT1 and ACT2) were prospective, double-blind, placebo-controlled, randomized studies where researchers compared the pain reduction capabilities of GammaCore against a placebo. Here are the core findings from those studies:
ACT1 involved 85 patients with episodic cluster headaches and found that 34.2% of patients experienced a reduction in pain from episodic cluster headache.
This reduction was defined as:
“the percentage of patients who reported mild or no pain 15 minutes after treatment initiation with GammaCore for the first treated cluster headache attack”.
In the placebo group, only 10.6% of patients recovered.
ACT2 involved the analysis of 182 attacks in 27 patients with episodic cluster headache. That study also found that a significantly higher percentage of attacks were pain-free (defined as pain-free at 15 minutes after the onset of pain) after being treated with GammaCore. Specifically, 47.5% of the GammaCore group and 6.2% of the placebo group were pain-free within 15 minutes.
Importantly, GammaCore was found to be safe and well-tolerated in all studies. The majority of adverse events were “mild and transient and occurring during the time of active treatment”.

What To Expect During GammaCore Treatment
GammaCore is a non-invasive, handheld medical device. You apply it to the neck to acutely treat the pain associated with cluster headache.
The device is designed to be portable and easy-to-use. You can self-administer treatment at home – or wherever you go.
To use GammaCore, you just place on your neck over the vagus nerve. Then, GammaCore stimulates the nerve’s afferent fibers in order to modify pain signals.
About ElectroCore
ElectroCore, LLC is an American bioelectric medicine healthcare company best-known for its vagus nerve stimulation technology – like the technology used in GammaCore. The company is developing vagus nerve stimulation technology for the treatment of a variety of conditions – including neurology, psychiatry, gastroenterology, and more.
It can be used for more than just cluster headache.
ElectroCore was founded in 2005. Today, they have more than 30 employees and full-time consultants, including offices in the United States (headquarters), Germany, UK, Italy, Australia, and Canada.
In 2010, ElectroCore scientists experienced a breakthrough in their non-invasive delivery technology that “allowed the company to expand beyond the acute emergency indications and realize the full breadth of potential of acute and prophylactic vagus nerve stimulation”, explains the official website. GammaCore was introduced to markets shortly thereafter.
How To Buy GammaCore
GammaCore is in use outside of the United States, including in the European Union. It’s also released, cleared, licensed, or approved in Canada, Australia, Colombia, Hong Kong, India, New Zealand, South Africa, and Vietnam.
Stateside, GammaCore should be available early in Q3 2017. Watch for GammaCore to appear on store shelves in the United States before the end of 2017!